Ground State Electron Configuration Chart
Answer 1 of 5.
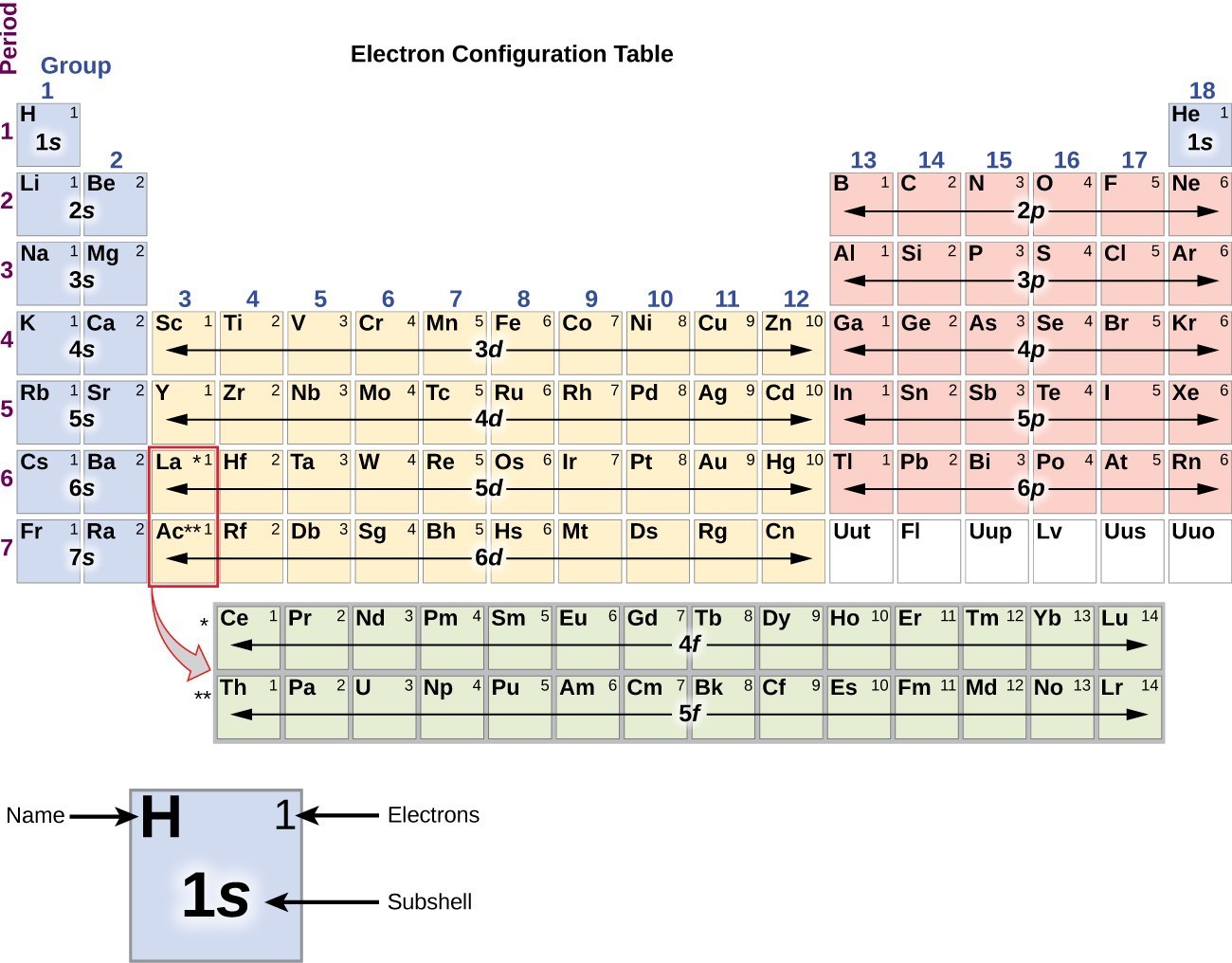
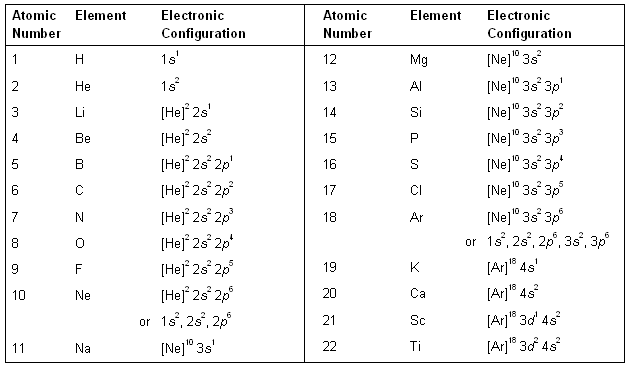
Ground state electron configuration chart. 105 rows The electron configuration shows in which states individual electrons are located. The ground state electron configuration of ground state gaseous neutral titanium is Ar. The electron configuration of Nitrogen 7N will have 2 electrons in the innermost shell 1s and 5 electrons in the next shell which is divided into two subshells 2s and 2p.
The Aufbau Principle. This article provides you with an electronic configuration chart for all these elements. Cesium barium lanthanum.
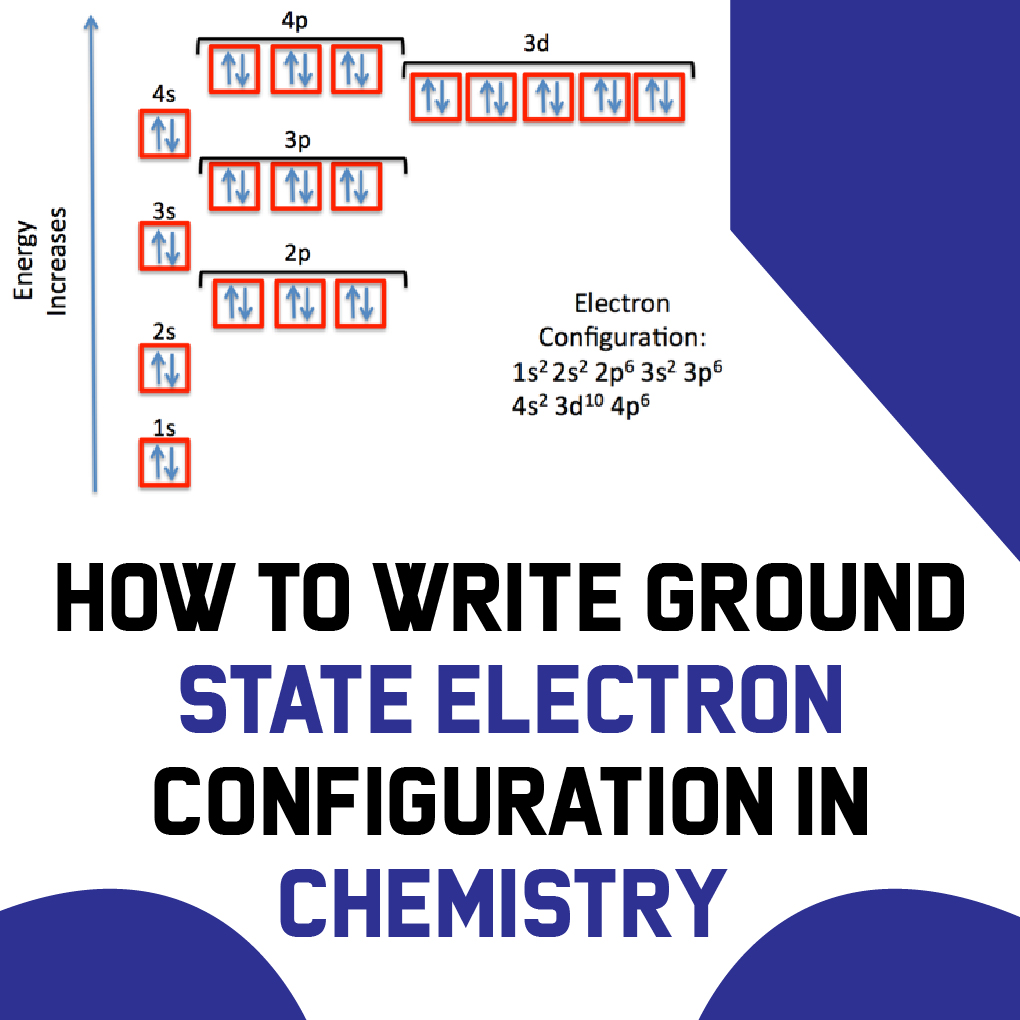
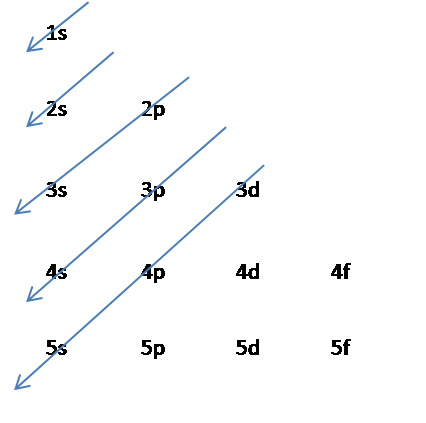
However one can write the electronic configuration just by understanding the Aufbau principle. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 This video explains how to use the Aufbau principle and a diagonal diagram to write electron configurations. It is a mnemonic used to remember the order of filling of atomic orbitals during the construction of the ground state electron configurations of the elements.
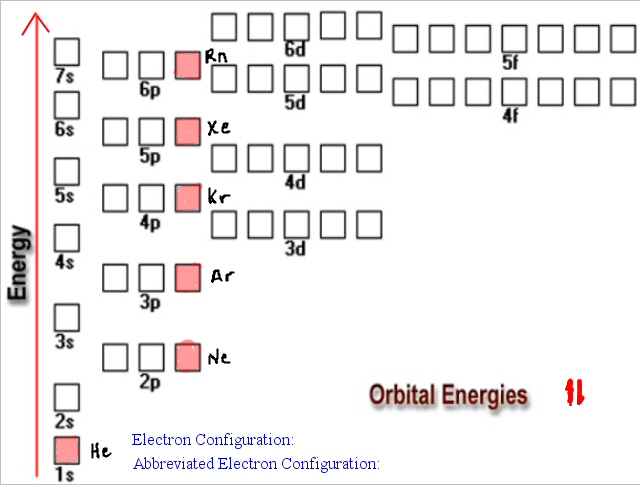
Rn 5f 14 6d 10 7s 2 7p 6. The n l Rule. Its electron configuration is.
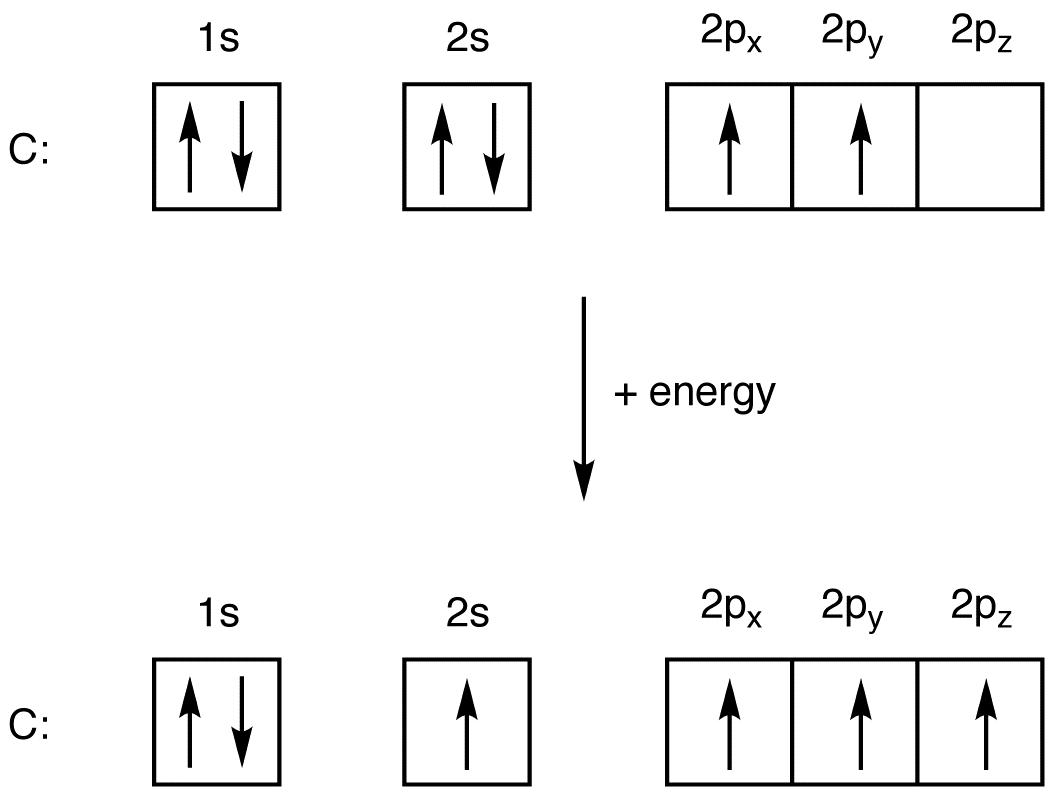
Now find the atomic number of the first. Here are a number of highest rated Electron Configuration On Periodic Table pictures upon internet. The above diagram roughly depicts the relative energy difference between these three ways of filling 2 electrons into the three p orbitals.
Keep in mind electron configurations are most stable when they are filled or half-filled. Zirconium atoms have 40 electrons and the shell structure is. Identify the ground state using Hunds rule maximum multiplicitymaximum of parallel spins results in lowest e-- e-repulsion.
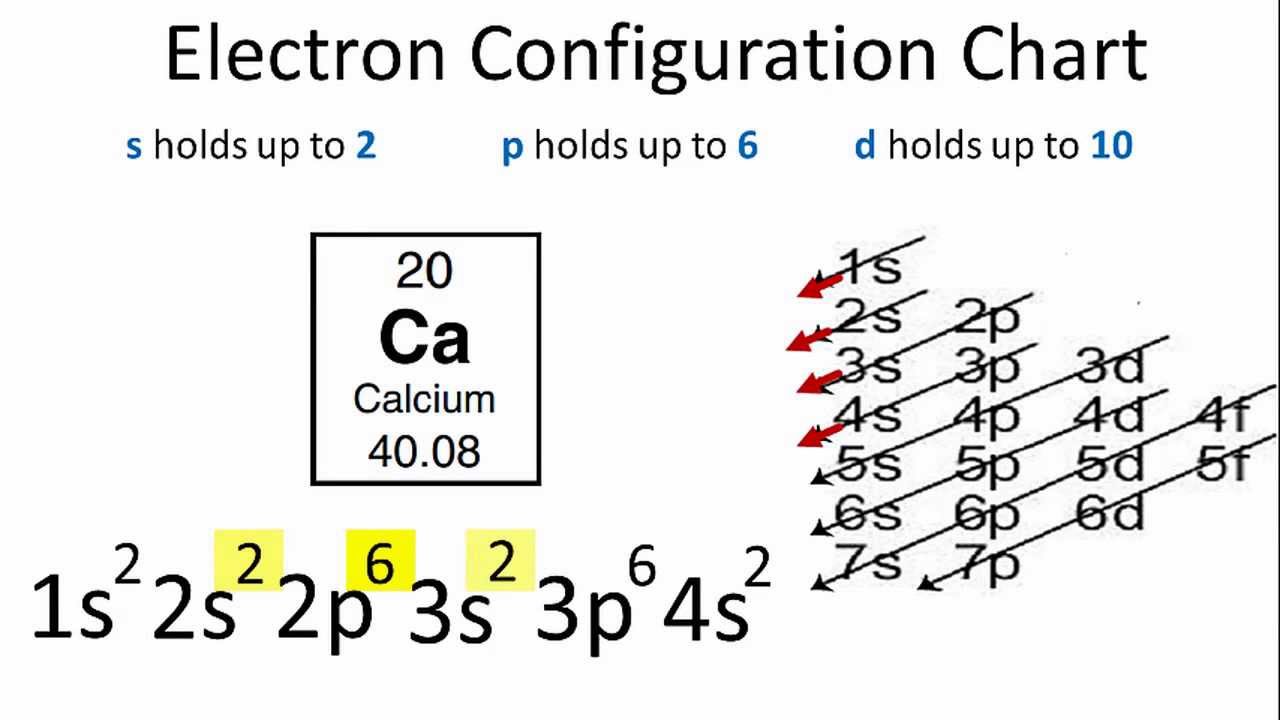
Its submitted by admin in the best field. Higher the value of nl for the orbital higher is the energy. For example calcium is element 20.
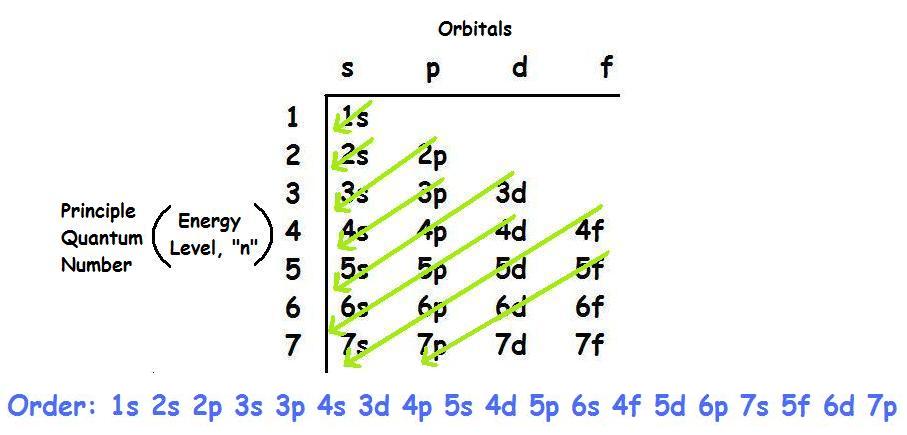
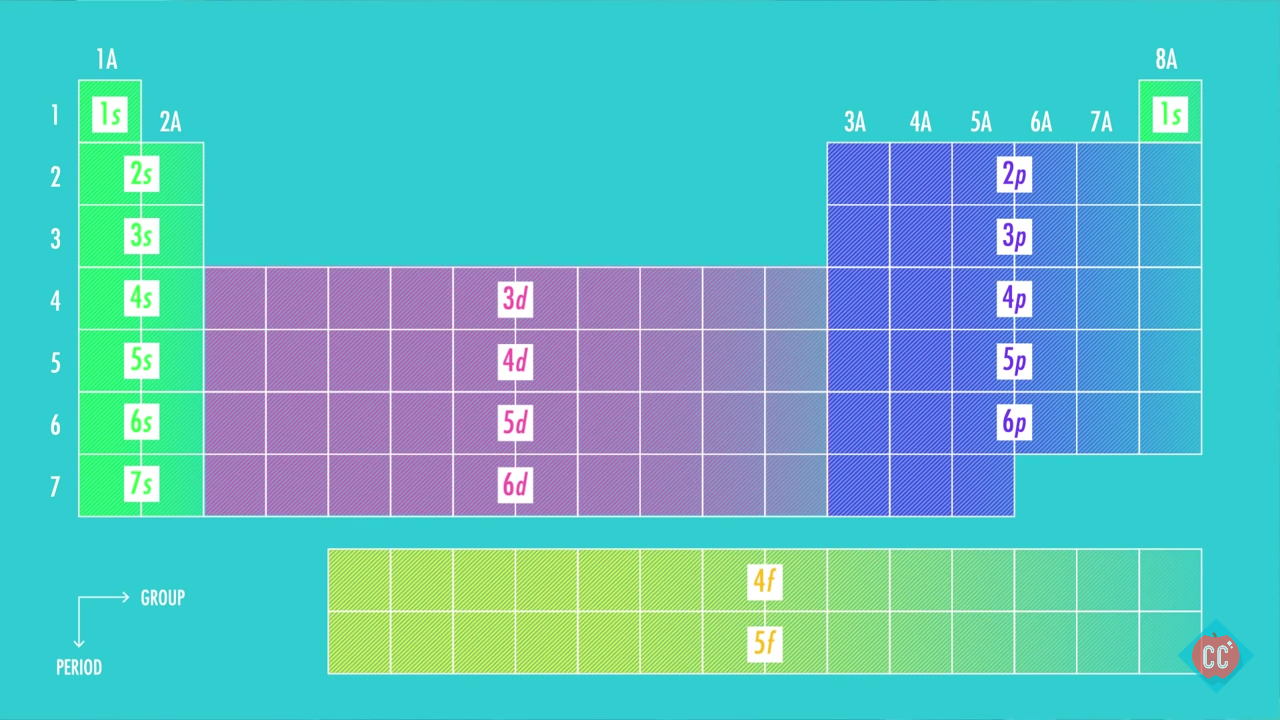
It states that in ground state the electrons occupy the atomic orbitals in their order of increasing energies which is given by nl rule. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6 7s 2 5f 14 6d 10 7p 6. Once you practice a few different examples writing electron configurations will become pretty easy.
Our free-electron configuration calculator also depicts an abbreviated way of finding electron configuration. The presentation of this diagram is largely disconnected from any physical. 1s22s22p x 12p y 1 or 1s22s22p x 12p z 1 or 1s22s22p y 12p z 1.
We identified it from trustworthy source. 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁵ Still since 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ is the electronic configuration of the last previous noble gas. Weve all seen and use the so-called Aufbau Diagram Figure 1.
The ground state electron configuration is the arrangement of electrons around the nucleus of an atom with lower energy levels. 4s2 and the term symbol is 3F2. There are 53 electrons occupying the respective orbitals as follows.
We take this kind of Electron Configuration On Periodic Table graphic could possibly be the most trending subject once we allowance it in google gain or facebook. Alternatively write the symbol for the noble gas before an element radon in this case and just add the extra information. So based on what we know about the quantum numbers and using the chart above you need 2 electrons to fill an s orbital 6 electrons to fill a p orbital 10 electrons to fill a d orbital and 14 electrons to fill the f orbital.
Full electron configuration of barium. If you want to do manually then follow the steps below to write shorthand electron configurations. 119 rows Shorthand Electron Configuration Full Electron Configuration.
The electrons occupying the orbitals of varying energy levels. What is the ground state electron configuration for Element 40. The electronic configuration of each element is decided by the Aufbau principle which states that the electrons fill orbitals in order of increasing energy levels.
Electronic configuration of the neutral Iodine atom. Which element has the following ground state electron configuration. You are watching.
This video might also be useful it describes how to write short-cut electron configurations. First find the required element on the periodic table. BUT what we havent.
What is the electronic configuration of 7N.









/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)