Cations And Anions Chart
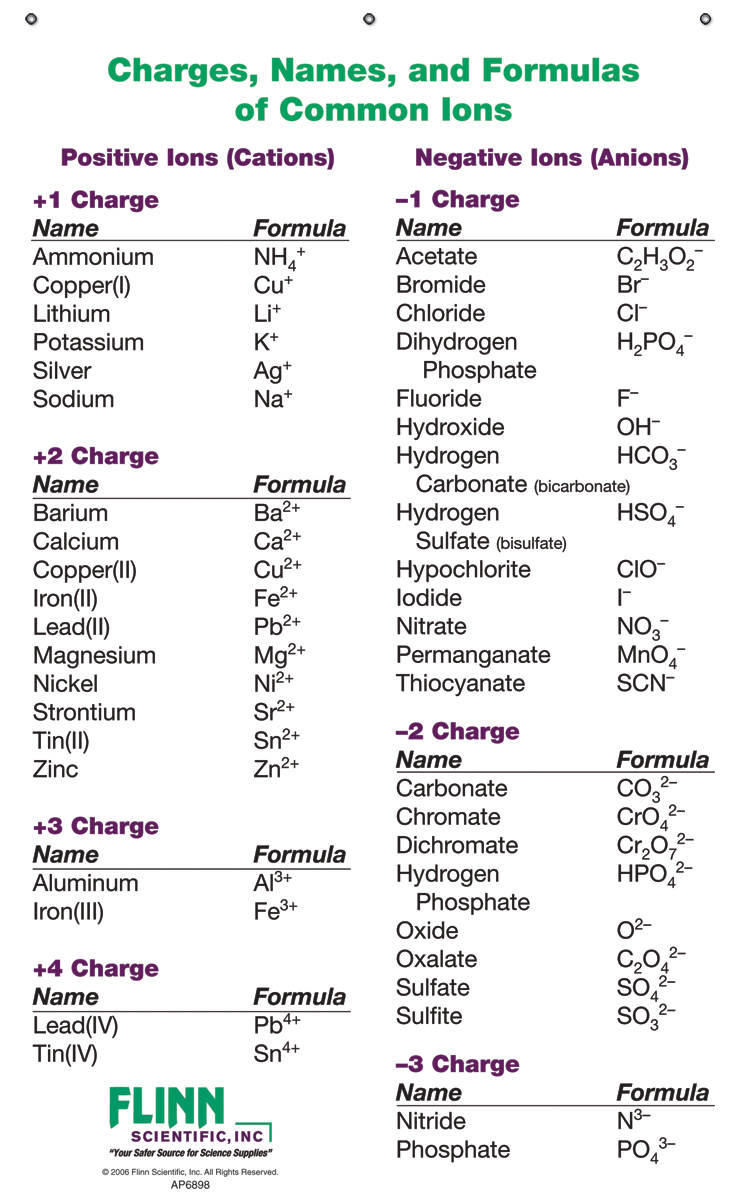
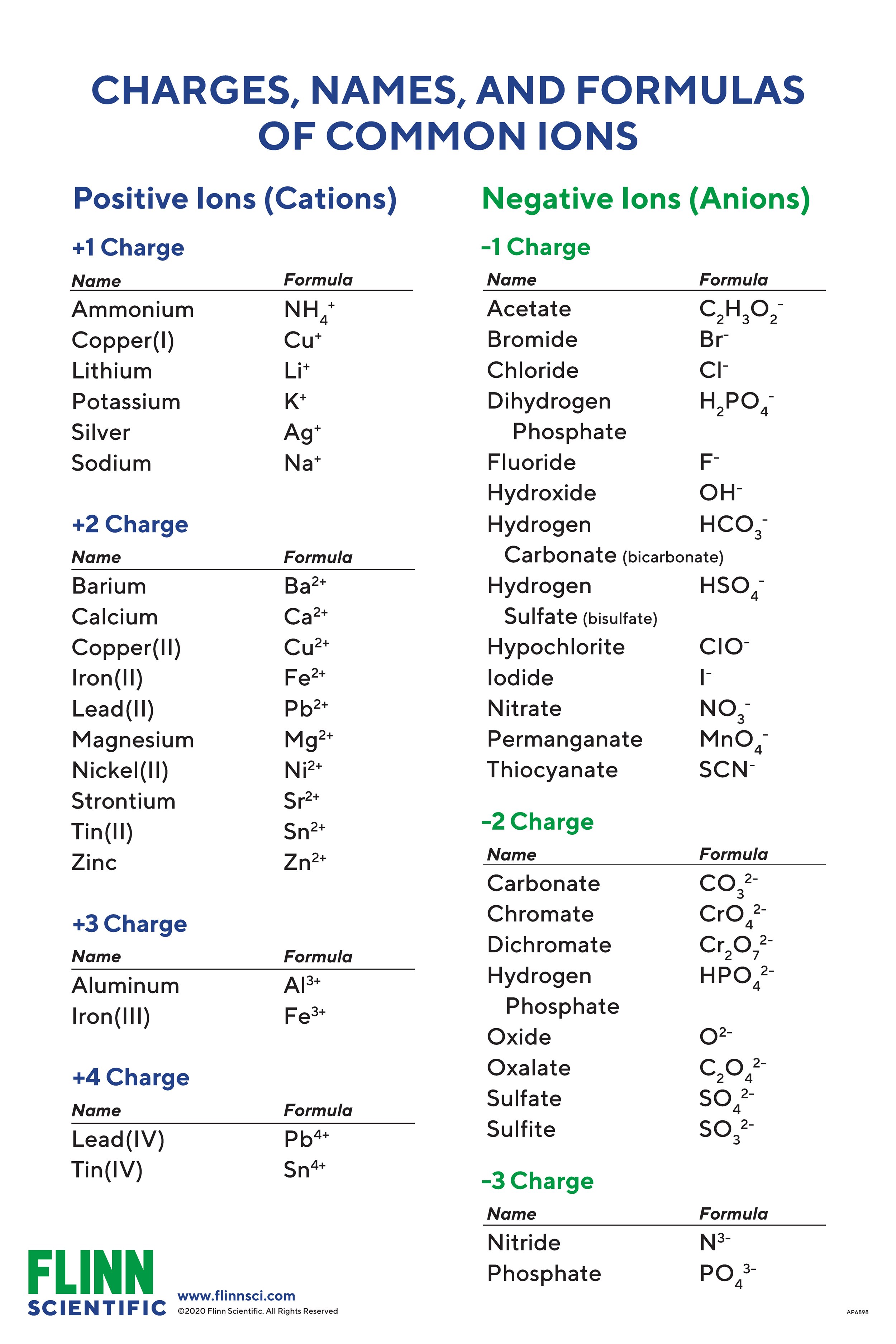
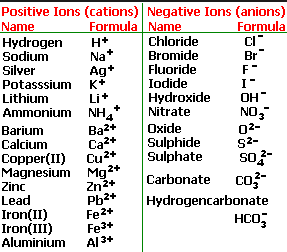
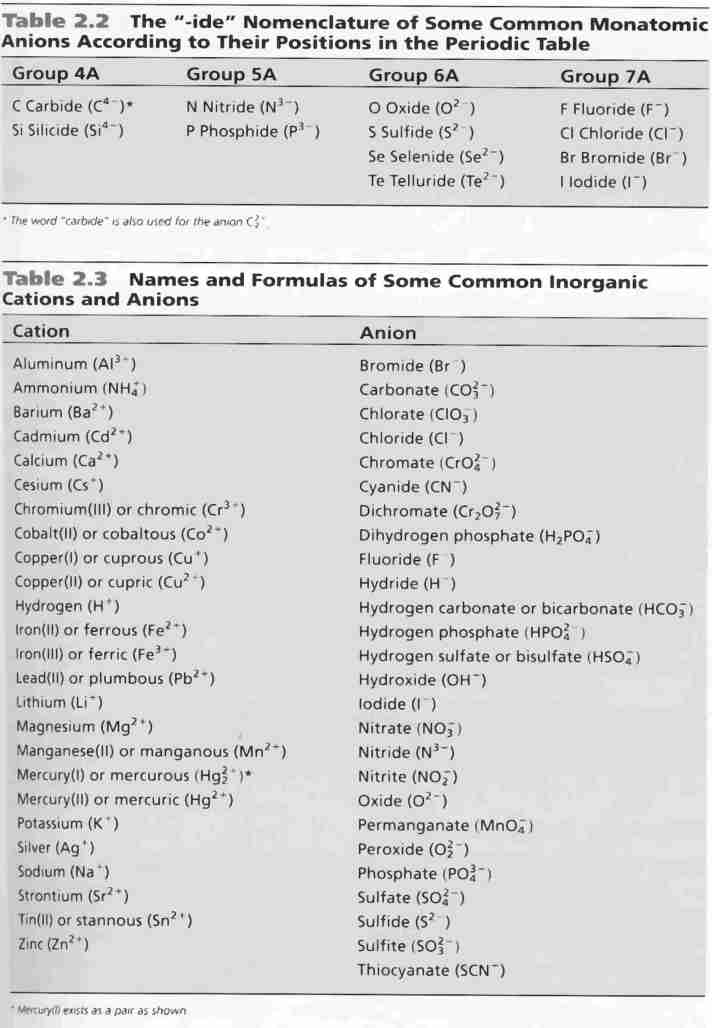
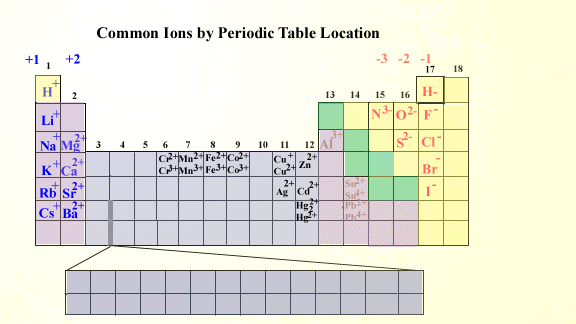
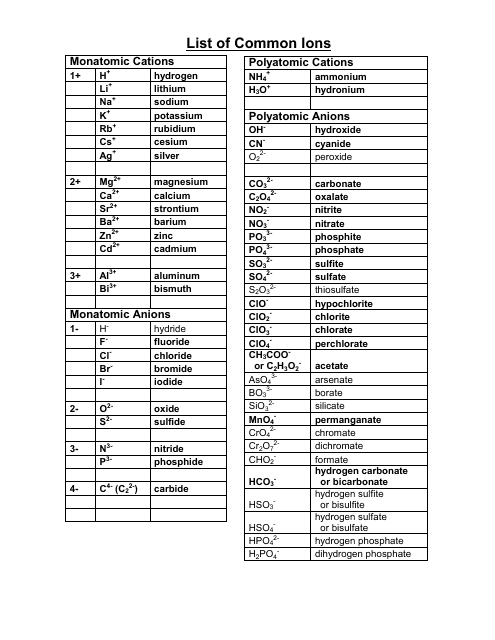
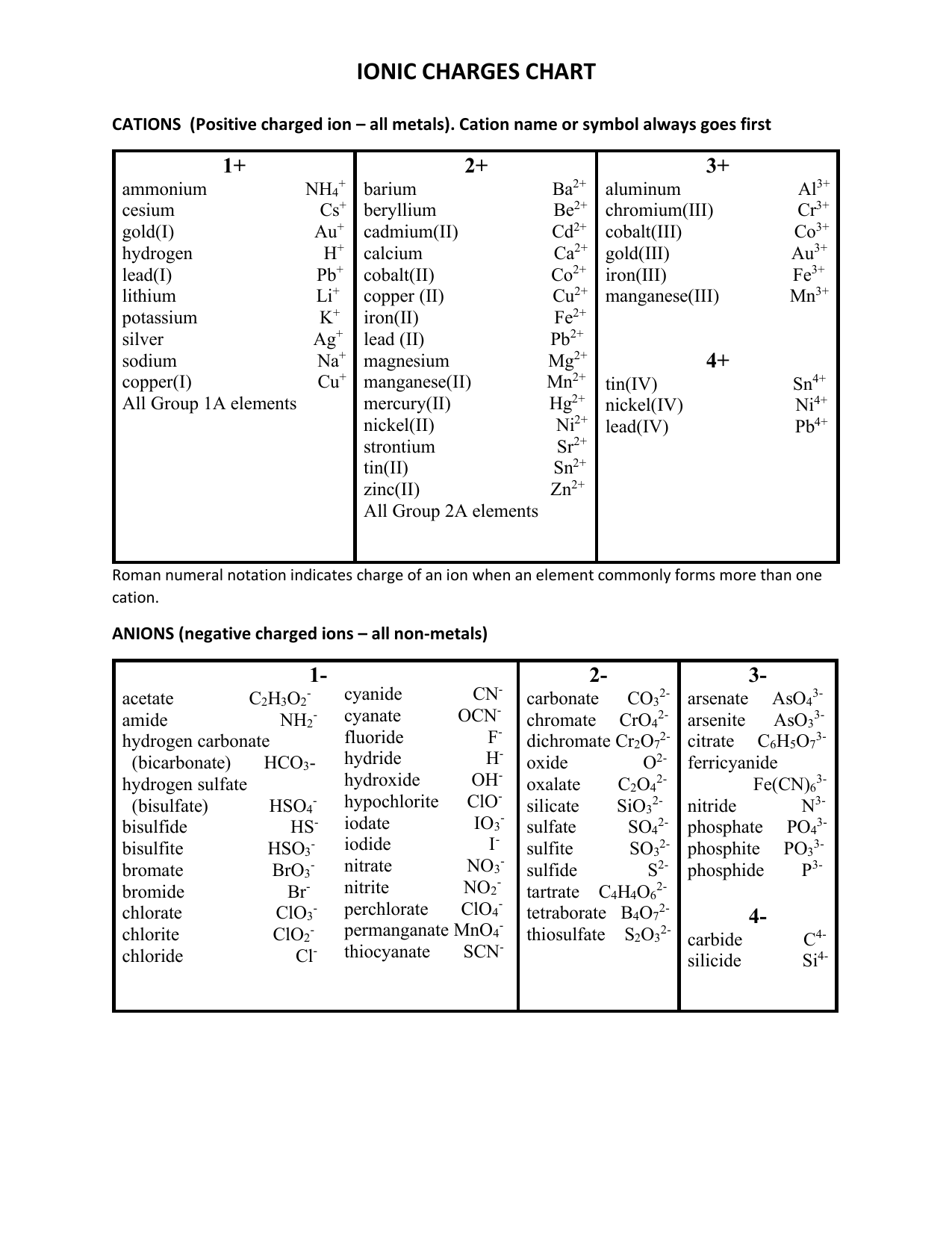
Ionic Charges Chart Cations and Anions Cations 1 ammonium NH 4 cesium Cs goldI Au hydrogen H leadI Pb lithium Li.
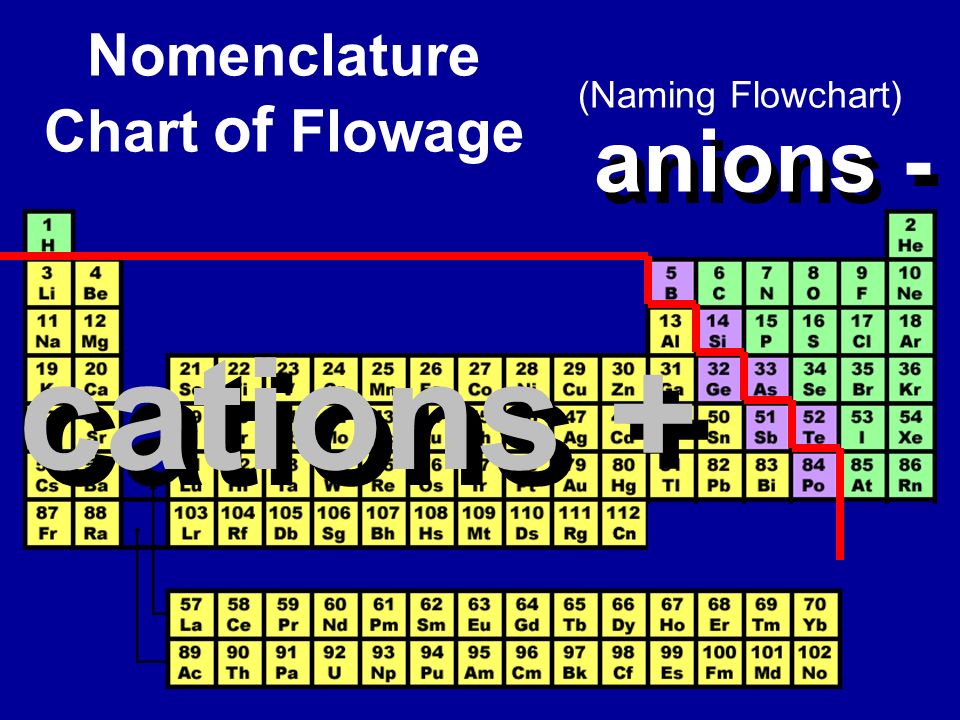
Cations and anions chart. Halogens always form anions alkali metals and alkaline earth metals always form cations. It can be possible to predict whether an atom will form a cation or an anion based on its position on the periodic table. Qualitative Analysis of Cations and Anions Objectives.
However theses are salts of weak acids and will all dissolve in acidic solution. The addition of HCl in the second step fails to dissolve BaSO 4 since SO 4 2-is the anion of a strong acid. Cation mAnion nH 2O If 0 H 2O is omitted Examples Cations on the left anions on the right charge must cancel Strontium Chloride Strontium ions and Chloride ions Sr2 and Cl SrCl 2 Multiples of polyatomic ions require parenthesis.
The results of these steps are summarized in the flow chart below. 1 Experiment 12. Ion Chart Periodic Table.
Most other metals form cations eg. In each question a test is. In other words write the cation on the left and the anion on the right.
To perform qualitative analysis of two unknown solutions that contain various ions cations and. Where the H 2 O is omitted if the is zero m is the oxidation state of the anion and n is the oxidation state of the anion. Anions and cations are both ions.
Use the flow charts on the previous page to answer the following questions. Table 1 General Properties of Cations Color of Flame Test Color of Name Formula Aqueous Soln Color Salts 1. Iron silver nickel whilst most other nonmetals typically form anions eg.
Qualitative Analysis of Cations Pre-Laboratory Assignment The pre-lab assignment for Part A of the experiment is to complete the flow chart Qualitative analysis of cations and anions pdf analysis of cations and anions qualitative analysis to identify ions such identifying four common anions in A system of qualitative analysis for the. They have an opposite electrical charge therefore they get attracted to each other. Transition Elements On Periodic Table.
Monatomic Cations and Anions Symbol H Li Na K Rb Cs Pb4 Mg2 Ca2 Co2 Ba2 Ag Ra2 Ni2 Zn2 Cu Name hydrogen ion lithium ion sodium ion potassium ion rubidium ion cesium ion lead IV plumbic magnesium ion calcium ion cobalt II cobaltous barium I. Cationanion prefixhydrate If 0 hydrate is omitted Formula. Chemistry Ion Chart Chart of Common IonsIonic Charges POSITIVE IONS CATIONS NEGATIVE IONS ANIONS Aluminium Al3 Acetate CH 3COO-Ammonium NH 4 Bromide Br -Barium Ba2 Carbonate CO 3 2-Cadmium Cd2 Hydrogen carbonate bicarbonate HCO 3-Calcium Ca2 Chlorate ClO 3-Chromium II Cr2 Chloride Cl -Chromium III chromic Cr3.
1CationA positively charged ion is known as cationA cation is formed by loss of one or more electrons by an atom. Because of their net electrical charge cations are repelled by. Due to more protons than electronsa cation has positive charge on it.
Other anions also form insoluble precipitates with barium ions such as BaCO 3 BaSO 3 and Ba 3PO 4 2. Cations are distinguished from adding sodium hydroxide and aqueous ammonia while testing of anions mainly involve precipitation. CalciumII ion Ca 2 colorless red-orange colorless.
CopperII ion Cu 2 blue or deep blue depends on cupric ion green or green anion 2. The number of protons is more than the number of electrons in a cation whereas the number of electrons is more than the number of protons in an anion. Secondary School Chemistry This blog is dedicated to secondary school students taking Chemistry.
Cation repels other cation whereas anion repels another anion. Key Differences Between Cation and Anion. The net charge gained by an ion of an atom or atoms is the fundamental phenomena to separate the and anion.
Given below are the essential points which differentiate the cations to that of anions. Quliatative analysis for testing of cations anions and gases. LeadII ion Pb 2 colorless faint blue-gray some colored 3.
Table Of Cations And Anions. A cation is formed by removal of electrons from an atom therefore a cation contains less electrons than normal atom. Common Cations and Anions Name Formula Charge Name Formula Charge Name Formula Charge aluminum Al 3 3 magnesium Mg 2 2 carbonate CO 3 2 2 ammonium NH 4 1 manganese II Mn 2 2 chlorate ClO 3 1 barium Ba 2 2 manganese III Mn 3 3 chloride Cl 1 cadmium Cd 2 2 mercury I mercurous See note Hg 2 2 2 X 1 chromate CrO 4.
A cation has fewer electrons than protons. To understand the rationale and the procedure behind the separation for various cations and anions. Cations are ions which have a positive electrical charge.
The formula of a salt is. So an atom or molecule having more number of protons than electrons and are positively charged is called cation while an. Main Group Periodic Table.
I hope that through the use of the resources here you will. Cation m anion n H 2 O. If m or n is 1 then no subscript is written in the formula.